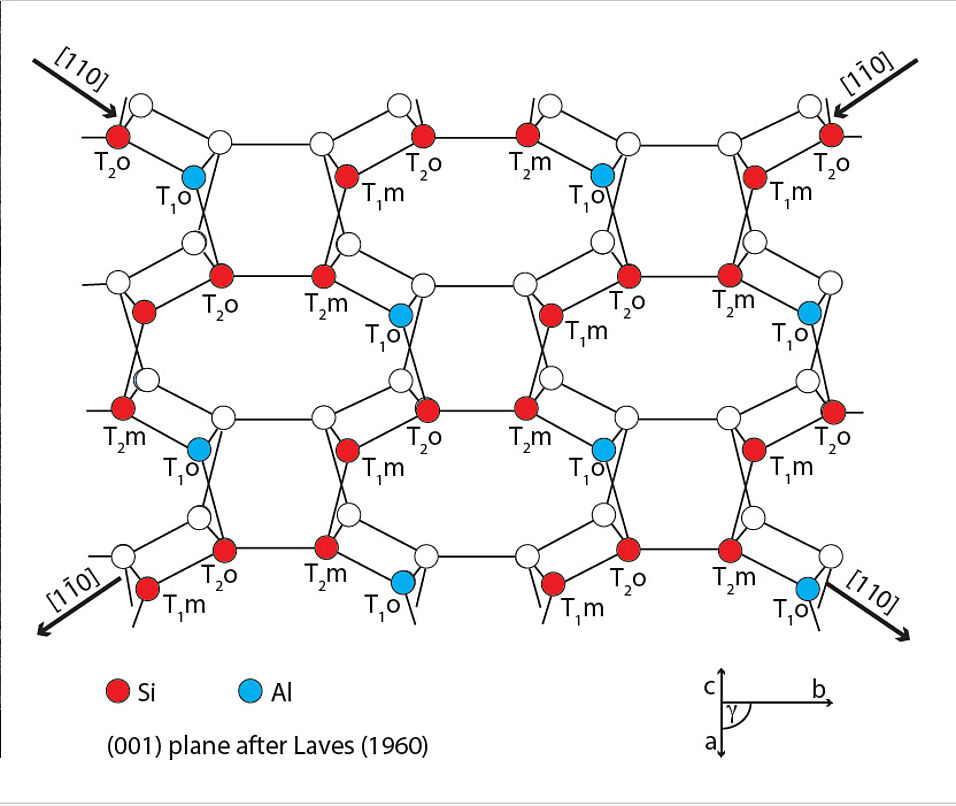
Feldspar is the most common mineral in the Earth's crust, constituting about 50% of its volume. But Feldspar not only paves the ground, it is also found high up in the sky, where it forms about 10% of the airborne mineral dust and supposedly plays an important role in the formation of ice in tropospheric clouds.
Which role and how important it is, was topic of the Feldspar Days in April 2025 at the Department of Lithospheric Research in Vienna. The petrology working group at the department is involved in cloud microphysics studies on ice nucleation on feldspar. "What started as a bilateral FWF-DFG project between the Karlsruhe Institute of Technology and UNIVIE has become a multilateral, highly interdisciplinary international group sharing the aim to unravel the mechanisms underlying the extraordinary ice-nucleating activity of alkali feldspar", says Rainer Abart, head of the Petrology group and Dean of the Faculty of Earth Sciences, Geography and Astronomy.
Aim to unravel the mechanisms underlying the extraordinary ice-nucleating activity of alkali feldspar
From 24th to 25th April, 25 researchers from all over Europe gathered at our faculty to discuss the different approaches of addressing ice nucleation on feldspar surfaces, including targeted freezing experiments done at the Department of Lithospheric Research and at the Karlsruhe Institute of Technology (KIT), Non Contact Atomic Force Microscopy done at TU Vienna and at University of Bielefeld which delivers atomic scale information on the adsorption of H2O molecules on feldspar surfaces, synchrotron experiments at the Hamburg Synchrotron Facility that allow for monitoring ice nucleation in real time, and atomistic modelling, which is performed at the Faculty of Physics, UNIVIE, at TU Vienna, and at the University of Helsinki.
Alkali feldspar aerosol particles raise ice nucleation temperatures
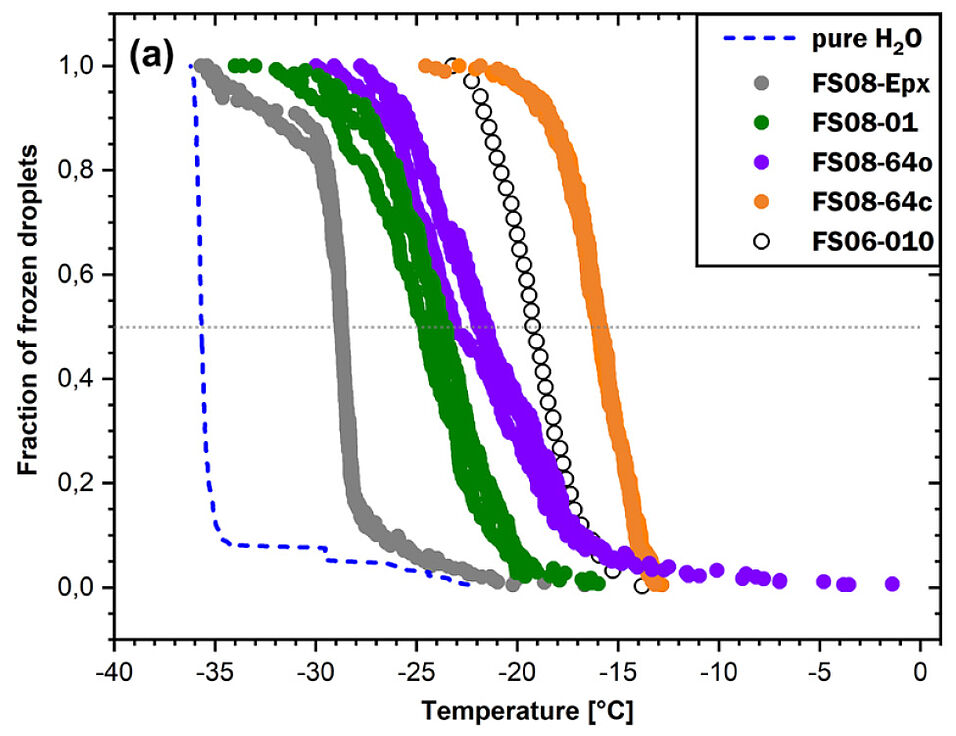
In the absence of aerosols, micrometer-sized water droplets suspended in air can be supercooled to about -37°C before homogeneous ice nucleation sets in. In the presence of aerosols, the suspended droplets freeze anywhere between 0◦C and -37◦C. Aerosol particles of alkali feldspar, a subgroup of the feldspar minerals, are particularly efficient in raising ice nucleation temperatures.
Ice formation influences the physical properties of clouds
Ice formation influences the physical properties of clouds, including, among others, their reflectivity, with implications for the Earth’s radiation balance and climate. Moreover, ice particles serve as a substrate for the adsorption of trace gases and thus influence atmospheric chemistry. It is therefore of considerable interest to understand why alkali feldspar aerosol particles exhibit their extraordinary ice-nucleating properties.
Another example of how the diversity in our faculty can lead to interesting new avenues of research
"Hosting the highly interdisciplinary Feldspar&Ice community at our faculty is another example of how the diversity in our faculty can lead to interesting new avenues of research", says Abart.